A newly expanded prostate cancer drug may carry new hope to sufferers with a typical type of the illness.
Novartis, a Switzerland-based pharmaceutical firm, introduced on March 28 that the U.S. Meals and Drug Administration (FDA) has expanded approval for Pluvicto (lutetium Lu 177 vipivotide tetraxetan), a focused radioligand remedy (RLT) that’s given earlier than chemotherapy.
(RLTs are a type of focused nuclear medication that docs use to deal with a number of types of cancer, in keeping with Novartis.)
PROSTATE CANCER RISK INCREASES BY 45% AMONG MEN WHO SHARE ONE TROUBLING BEHAVIOR
The drug is meant for sufferers with PSMA-positive metastatic castration-resistant prostate most cancers (mCRPC) who’ve obtained one spherical of androgen receptor pathway inhibitors (ARPIs), a category of medication used within the therapy of metastatic prostate most cancers.

A newly expanded prostate most cancers drug may carry new hope to sufferers with a typical type of the illness. (iStock)
Pluvicto first acquired FDA approval on March 23, 2022, however this new expanded approval triples the variety of sufferers eligible to receive the drug, in keeping with a Novartis press launch.
The drug is run by an IV into the bloodstream, the place it attaches to prostate most cancers cells and both retains them from replicating or kills them.
PROSTATE CANCER CASES SPIKE IN THIS US STATE AS DOCTORS SHARE LIKELY REASON
“The sooner indication for Pluvicto may actually change our therapy paradigms for sufferers with mCRPC,” stated lead research creator Michael Morris, MD, prostate most cancers part head at Memorial Sloan Kettering Most cancers Heart in New York.
“It gives a focused remedy that higher delays illness development in comparison with a second ARPI. This approval is a big step ahead and may open the doorway to a remedy that has clear medical benefits for the affected person with mCRPC who has progressed on one ARPI and has not obtained chemotherapy.”
In medical trials, Pluvicto “considerably decreased the chance of development or dying” by 59%.
This can be a type of prostate most cancers that has unfold to different components of the physique and doesn’t reply to straightforward hormone remedy, in keeping with WebMD.
It additionally has excessive ranges of prostate-specific membrane antigen (PSMA), a protein produced by prostate most cancers cells.
In clinical trials, Pluvicto “considerably decreased the chance of development or dying” by 59% in mCRPC sufferers, Novartis reported.
IMAGE
“The FDA’s expanded approval of [lutetium Lu 177 vipivotide tetraxetan] marks a transformative step ahead within the therapy of mCRPC, underscoring the rising affect of precision oncology,” Jorge A. Garcia, MD, a genitourinary medical oncologist and chair of the Strong Tumor Oncology Division at College Hospitals Seidman Most cancers Heart/Case Western Reserve College in Cleveland, Ohio, advised OncLive.
CLICK HERE TO GET THE FOX NEWS APP
“By enabling entry to this focused radioligand remedy previous to chemotherapy, we’re not solely broadening therapy choices, but additionally redefining the usual of take care of PSMA-positive illness.”
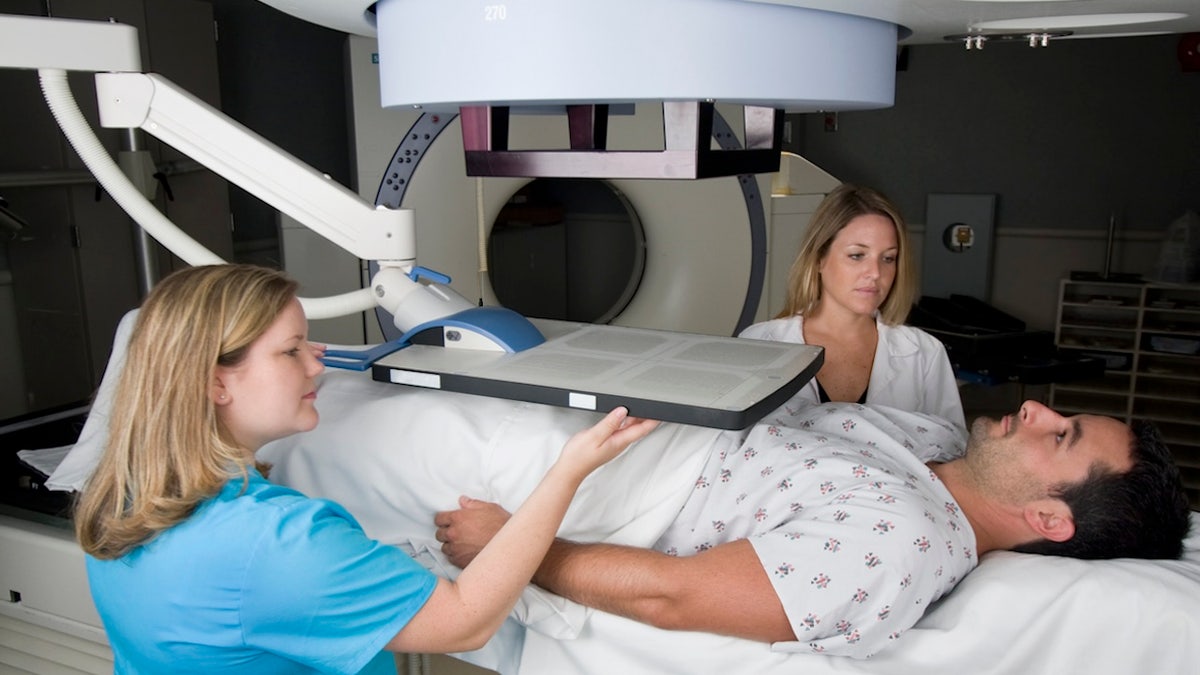
“By enabling entry to this focused radioligand remedy previous to chemotherapy, we’re not solely broadening therapy choices, but additionally redefining the usual of take care of PSMA-positive illness,” a researcher stated. (iStock)
Prostate most cancers is the second main explanation for death among men; mCRPC makes up a majority of the deaths and 20% of all metastatic prostate most cancers instances.
Research have proven that roughly 10% to twenty% of sufferers with prostate most cancers develop mCRPC inside 5 years of follow-up after preliminary remedy, and instances of metastatic sufferers have risen 4% to five% every year since 2011.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Sixty p.c of prostate cancers are recognized in males who’re 65 or older, in keeping with the American Most cancers Society. The danger of being recognized with metastatic prostate most cancers sometimes happens between 65 and 74.
Antagonistic results of Pluvicto included dry mouth (61%), fatigue (53%), nausea (32%) and constipation (22%), the discharge acknowledged.
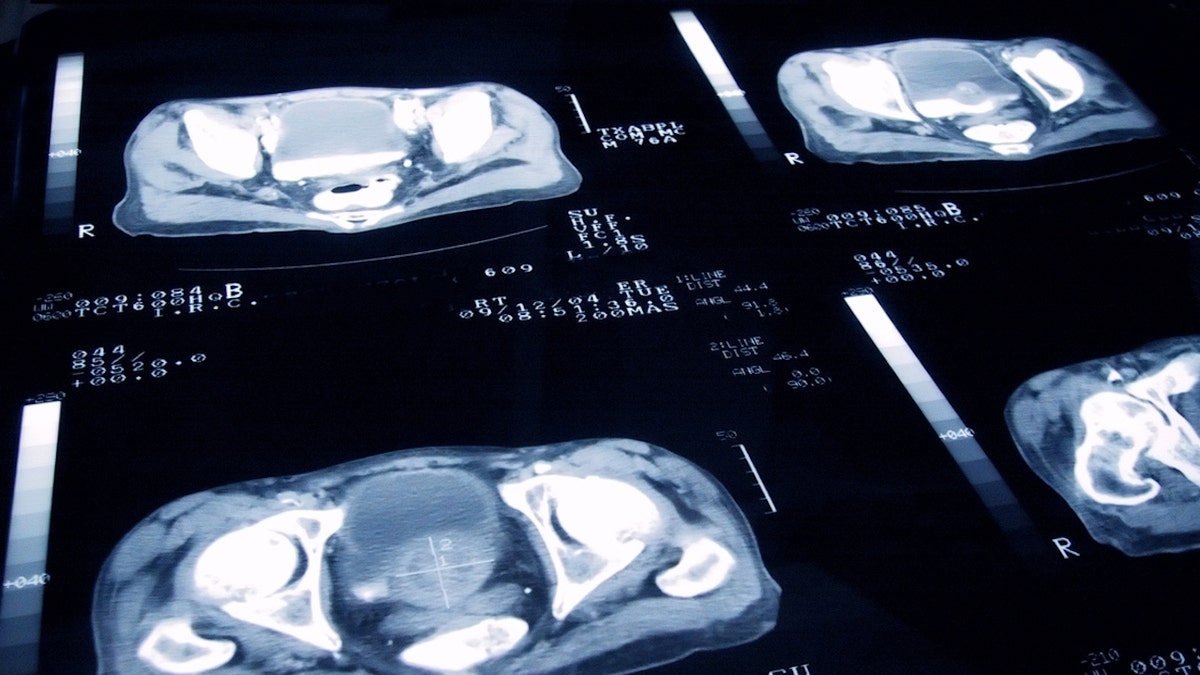
Prostate most cancers is the second main explanation for dying amongst males; mCRPC makes up a majority of the deaths and 20% of all metastatic prostate most cancers instances. (iStock)
The sufferers receiving the drug have been capable of proceed with chemotherapy after taking it.
Novartis is dedicated to delivering Pluvicto to the almost 600 RLT therapy websites within the U.S., the corporate acknowledged.
For more Health articles, visit www.foxnews.com/health
Wanting forward, Novartis stated it plans to research using RLTs for different forms of superior cancers, together with breast, colon, neuroendocrine, lung and pancreatic cancers.

