NEWNow you can hearken to Fox Information articles!
An experimental drug may assist to enhance motion for sufferers with spinal twine accidents.
NVG-291, an injectable peptide, has been examined in a phase 2 trial with eligible sufferers — a few of whom famous outstanding outcomes.
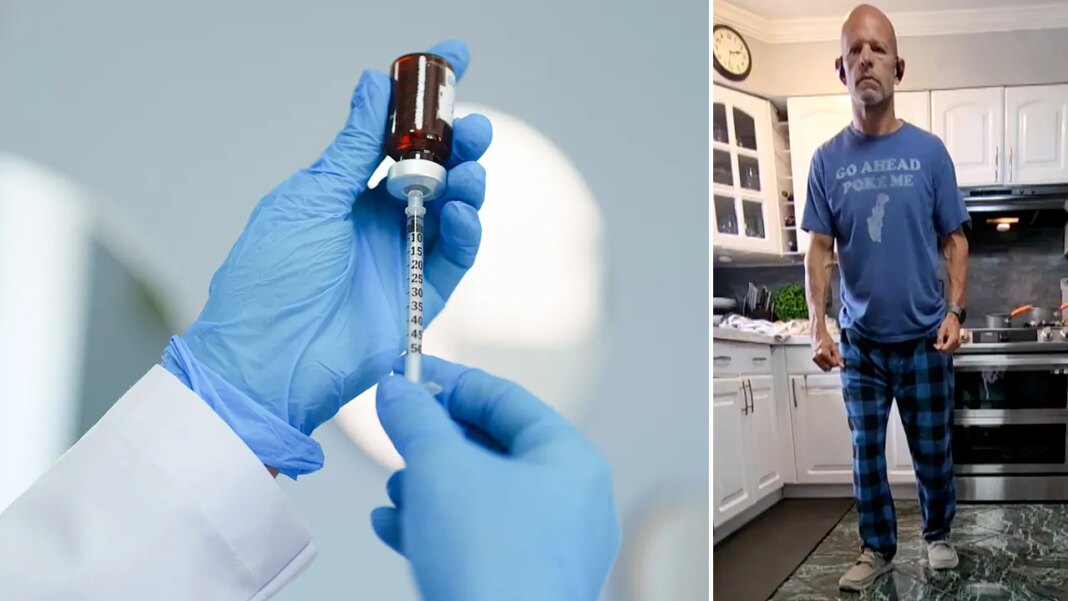
Larry Williams, a trial participant primarily based in Philadelphia, Pennsylvania, shared with Fox Information Digital that he’s been capable of stroll once more after an accident that prompted paralysis.
FOUR FAMILIES ARE DESPERATE TO RAISE FUNDS FOR THEIR CHILDREN’S PARALYZING DISEASE
Williams, 58, was mountain biking on a small path when he struck a tree. Though he was carrying a helmet, he “immediately broke” his C4 to C6 vertebrate (particular bones within the cervical backbone).
He underwent spinal surgery, however was paralyzed for 2 weeks till his physique started to “get up” and regained some motion after beginning remedy.

Larry Williams of Philadelphia (pictured) survived a mountain biking accident that left him paralyzed. (Larry Williams)
Williams was capable of stroll “just a little bit” with the help of a walker, however nonetheless had issues with mobility in areas like his arms. He additionally misplaced 40 kilos after the accident.
After some analysis, he found the NVG-291 trial and was thought of a viable candidate, he advised Fox Information Digital.
For 3 months, beginning in April 2024, Williams acquired a day by day injection of the drug adopted by one hour of physical therapy, which included hand workouts and strolling with a harness on a flat observe or treadmill.
NEW WEEKLY INJECTION FOR PARKINSON’S COULD REPLACE DAILY PILL FOR MILLIONS, STUDY SUGGESTS
Williams additionally underwent blood exams and electrophysiological testing to measure {the electrical} exercise of his nerves and muscle mass, in addition to bodily testing as soon as a month.
On the finish of the trial, Williams reported that he was capable of stroll 10 meters (32.8 toes), balanced with a walker, in 15 seconds, an enchancment over 45 seconds.
Though he has not acquired the drug since July 2024, Williams continues to see physical improvements over a 12 months later.
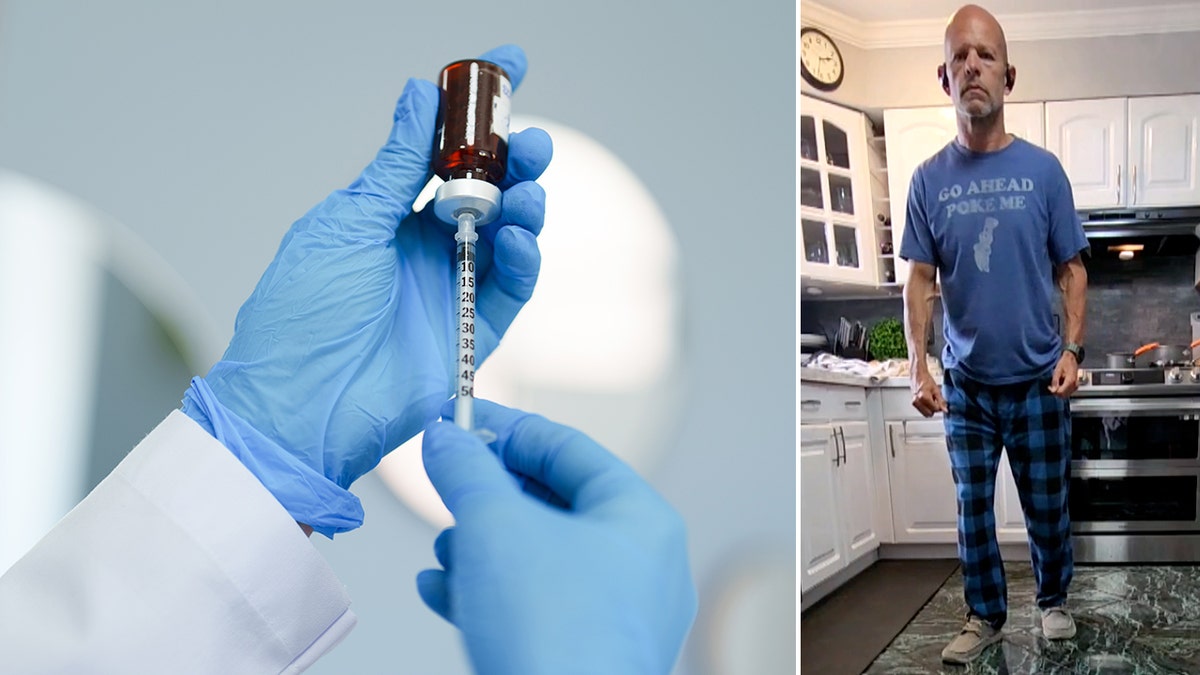
Larry Williams joined the NVG-291 trial as a spinal twine damage affected person in April 2024. (Larry Williams; iStock)
“I am not figuring out actually arduous. I am presently not in remedy,” he advised Fox Information Digital. “However simply a few days in the past, I stood up and tried to free-stand, stability and elevate one foot off the bottom. I used to be capable of do it for 30 seconds.”
“I hadn’t been training this. I am unable to clarify the way it occurred,” he added. “There are small enhancements that proceed to occur.”
Williams mentioned he had tried the identical maneuver six months earlier and couldn’t maintain his foot off the bottom for even three seconds.
“There are small enhancements that proceed to occur.”
Because the trial, Williams has continued to enhance his strolling means, and might even swim laps within the pool.
“I attain out to different individuals with the identical damage as me, and it looks like quite a lot of them, after years and years of remedy, get to the place I’m,” he mentioned. “And it sort of looks like I have been given a shortcut … I might like to get to the life that I had earlier than, being absolutely unbiased.”
WEIGHT-LOSS DRUGS NOW LINKED TO CANCER PREVENTION IN WOMEN, MAJOR NEW STUDY REVEALS
After taking the experimental drug, Williams mentioned he has been capable of carry out bodily duties “simply and rapidly.”
“The motion in my legs appears to be just a little bit smoother and fewer restricted because the time handed by,” he mentioned. “I am not going to surrender. I’ll hold pushing and making an attempt.”
How the drug works
In a separate interview with Fox Information Digital, lead researcher Dr. Monica Perez, scientific chair on the Shirley Ryan AbilityLab in Chicago and professor of bodily drugs and rehabilitation at Northwestern College, mentioned the drug was first examined on animals, which displayed improved locomotor perform (motion).
The researchers then carried out a randomized medical trial in people, through which half of the contributors acquired the NVG-291 drug, which contained a restore molecule to enhance nervous system function.
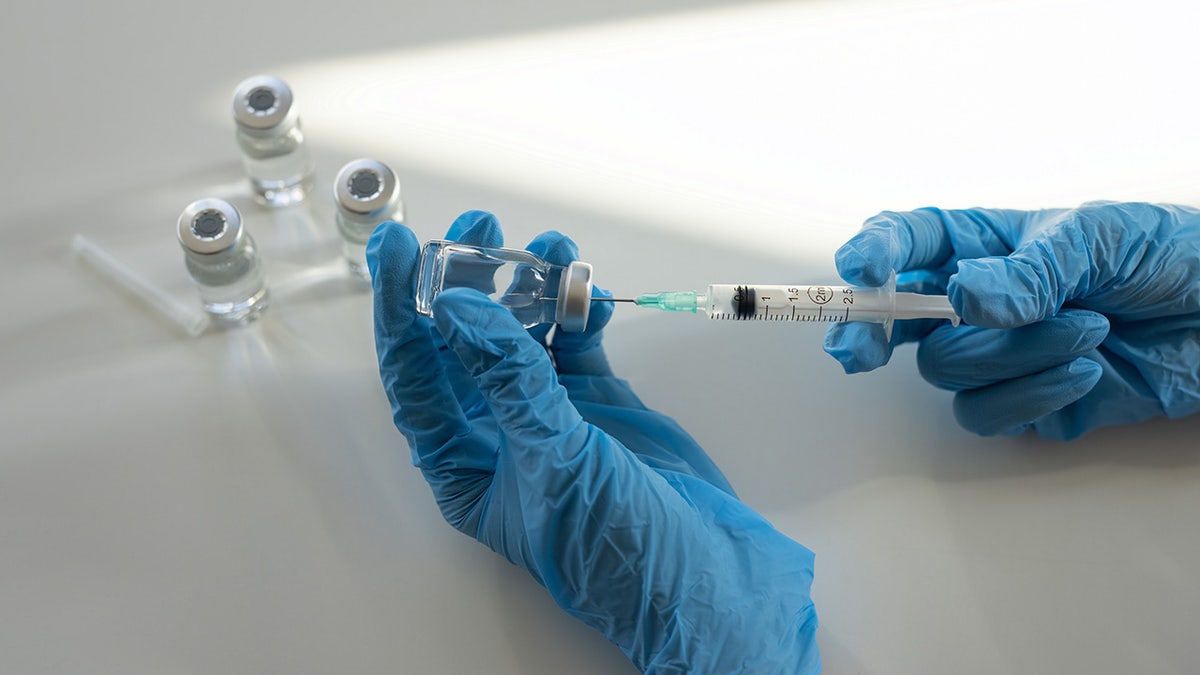
NVG-291 is a peptide drug that accommodates a restore molecule to enhance nervous system perform. (iStock)
The drug is a peptide, which is a small protein that works like a roadblock remover. After a spinal twine damage, the physique sends out alerts that inform nerve fibers to cease rising. This drug blocks these alerts, so the nerves have a greater probability to regrow.
“This peptide can block these inhibitory alerts,” Ryan mentioned. “There’s a little little bit of proof in animals that it will probably truly improve the expansion of neurons.”
CLICK HERE TO GET THE FOX NEWS APP
Though GLP-1 receptor agonists, finest identified for weight loss and diabetes therapy, are a peptide, Perez mentioned this spinal twine damage therapy works in another way.
“It has a particular mechanism, and it is extra associated to restore, to attempt to improve the expansion of neurons which might be affected by central nervous system damage,” she mentioned.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Spinal twine accidents are sometimes approached with cell therapies, like stem cells and bone marrow stromal cells, Perez famous.
This peptide method, in distinction, is “simple to manage,” might be finished at dwelling, achieves a “comparable aim” and is “extraordinarily protected,” she added.
Researchers noticed “electrophysiological adjustments” within the group that acquired the experimental treatment. (iStock)
Perez mentioned extra analysis is important to find out how lengthy the consequences of this drug will final.
“We noticed sturdy electrophysiological adjustments within the group that acquired the treatment in comparison with the placebo group, however we do not have follow-up measurements,” she mentioned. “There is no approach for us to know the true length of those remedies.”
As there may be not presently an FDA-approved therapy for spinal twine accidents, Perez mentioned that these concerned with this analysis are “very devoted” to advancing this science.
For more Health articles, visit foxnews.com/health
Whereas every affected person might react in another way, Williams mentioned he would advocate this therapy to different individuals with spinal twine accidents.
“It may actually change issues for individuals with accidents like mine,” he mentioned. “I am simply praying that everyone on the market is ready to have a chance to achieve again their life.”