When his toddler son was recognized with a rare, fatal disease, a Canadian father was dismayed to find there was no remedy or remedy. So he got down to make one himself.
Terry Pirovolakis, an IT director in Toronto, Ontario, welcomed his third son in Dec. 2017. It was a “regular, wholesome start,” he instructed Fox Information Digital — however inside six months, he and his spouse, Georgia Pirovolakis, observed their child, Michael, was not lifting his head.
“He simply didn’t appear to be he was assembly his milestones,” Pirovolakis stated.
After months of docs’ appointments, physiotherapy and genetic testing — what Pirovolakis describes as an “18-month diagnostic odyssey” — a neurologist recognized child Michael with spastic paraplegia 50 (SPG50), a neurological dysfunction that impacts fewer than 100 folks on the earth.
“They instructed us to simply go dwelling and love him — and stated he could be paralyzed from the waist down by age 10, and quadriplegic by age 20,” Pirovolakis stated.

When Michael Pirovolakis, pictured. was recognized with a uncommon, deadly illness as an toddler, his father, Terry Pirovolakis, was dismayed to find there was no remedy or remedy. That is when he got down to make one himself. (Terry Pirovolakis)
“They stated he’d by no means stroll or speak, and would wish help for the remainder of his life.”
What’s SPG50?
Spastic paraplegia 50 (SPG50) is a neurological dysfunction that impacts a child’s development, progressively resulting in cognitive impairment, muscle weak point, speech impairment and paralysis, in response to the Nationwide Group for Uncommon Problems.
Most individuals with the illness will die by the point they attain their 20s.
“Kids with SPG50 could expertise early developmental delays, muscle weak point and spasticity, however they proceed to try and adapt,” Dr. Eve Elizabeth Penney, an epidemiologist on the Texas Division of State Well being Companies and medical contributor for Drugwatch, instructed Fox Information Digital.
WHAT IS ANGELMAN SYNDROME? COLIN FARRELL’S SON IS LIVING WITH THIS RARE DISEASE
“Over time, these signs can worsen, making it onerous for affected people to stroll and carry out day by day actions,” added Penney, who was not concerned in Michael Pirovolakis’ care.
“The prognosis varies from individual to individual, however it’s usually a progressive situation, that means signs can turn out to be extra extreme over time,” she additionally stated.

Georgia Pirovolakis (left) is pictured together with her two sons, together with child Michael, who was recognized with SPG50. (Terry Pirovolakis)
Within the absence of a remedy, most households can solely handle signs by means of bodily remedy, occupational remedy, speech remedy and medications to help management spasticity or seizures, Penney stated.
“Managing SPG50 requires a complete, multidisciplinary method to deal with its numerous signs and challenges,” she added.
A father’s mission
There is no such thing as a remedy at present authorized by the U.S. Meals and Drug Administration (FDA) for SPG50.
After the shock of the prognosis, Pirovolakis instantly began researching, with a deal with discovering a gene therapy that might assist his son.
“They stated he could be paralyzed from the waist down by age 10, and quadriplegic by age 20.”
A month after his child’s prognosis, Pirovolakis flew to Washington, D.C., for a gene remedy convention, the place he met with a number of specialists. He additionally visited Sheffield, England, and the Nationwide Institutes of Well being on the College of Cambridge, the place scientists had been studying the disease.
“We then liquidated our life financial savings, refinanced our dwelling and paid a group on the College of Texas Southwestern Medical Heart to create a proof of idea to begin Michael’s gene remedy,” Pirovolakis stated.

Terry Pirovolakis, pictured along with his household, used his life financial savings to create a genetic remedy for his youngest son, heart, who has SPG50. (Terry Pirovolakis)
After profitable checks confirmed the gene remedy was efficient at stopping the illness’s development in mice and in human cells, Pirovolakis labored with a small drug firm in Spain to fabricate the drug.
On Dec. 30, 2021, Well being Canada granted approval to maneuver ahead with the gene remedy for Michael Pirovolakis.
STIFF PERSON SYNDROME PATIENTS SHARE WHAT IT’S LIKE TO LIVE WITH THE RARE DISEASE
“On March 24, 2022, my son was the primary particular person to ever get handled with gene remedy at SickKids in Toronto,” Pirovolakis stated.
The process, which includes injecting cerebral spinal fluid by means of a lumbar puncture, does include dangers — however the potential advantages are life-saving.
‘I couldn’t allow them to die’
After Michael Pirovolakis obtained the one-time remedy, there have been three extra doses left.
“We determined that we had to assist different youngsters,” Pirovolakis stated.
“Once I heard that nobody was going to do something about it, I needed to — I could not allow them to die.”

Pirovolakis’ two older youngsters, pictured with their little brother, Michael, backside left, wouldn’t have the illness. (Terry Pirovolakis)
Pirovolakis opened up a Section 2 research within the U.S., which treated three children two years in the past.
A kind of was 6-month-old Jack Lockard, the youngest baby to ever obtain the remedy.
“Jack has thrived since then,” Rebekah Lockard, the boy’s mom, instructed Fox Information Digital.
THE GIRL WHO CAN’T SMILE: HOW A RARE DISORDER BECAME A YOUNG WOMAN’S ‘GREATEST GIFT’
“He’s sitting independently, banging toys collectively, consuming from a straw cup and dealing actually onerous on crawling.”
She added, “Medical doctors and therapists share the identical sentiment: The remedy works!”
Different youngsters who participated in the trial have skilled related outcomes, Lockard stated.

The Lockard household, proven right here, is preventing to boost funds to acquire remedy for his or her daughter Naomi, at proper, who has SPG50. (Rebekah Lockard)
“They’ve all proven that their illness has stopped progressing and their cognition has improved.”
There are extra youngsters who nonetheless want the remedy — together with Lockard’s first baby, 3-year-old Naomi, who additionally has SPG50 — however are unable to entry it as a result of the medical trial has now run out of cash, as Fox Information Digital beforehand reported.
‘Time is of the essence’
It prices about $1 million to make the drug for every baby, Pirovolakis stated, and one other $300,000 or so to treat the patient within the U.S. on the hospital.
Pirovolakis has approached pharmaceutical corporations, however all of them have declined to fabricate the drug.
“We need to make sure that the trial strikes on and these youngsters get handled.”
“No investor goes to provide you cash to deal with a illness that’s not going to become profitable,” he stated. “That is the dilemma we’re in.”
Whereas Pirovolakis and his group are actively working to safe grants and buyers, it’s largely as much as the dad and mom to raise funds for the following part of the medical trial.

Up to now, Lockard has raised greater than $90,000 by way of GoFundMe (referred to as “Naomi and Jack Battle SPG50”) to get her daughter’s remedy, however that’s solely a fraction of what’s wanted. (Rebekah Lockard)
Up to now, Lockard has raised greater than $90,000 by way of GoFundMe (referred to as “Naomi and Jack Battle SPG50”) to get her daughter’s remedy, however that’s solely a fraction of what’s wanted.
Penney famous that remedy for SPG50 is difficult and costly to develop — “primarily as a result of it’s a sporadic illness.”
The physician instructed Fox Information Digital, “Pharmaceutical corporations typically prioritize situations that have an effect on bigger populations, with a extra vital potential for recouping analysis and growth prices.”
CHILDREN WITH TOTAL DEAFNESS REGAIN HEARING AFTER ‘GROUNDBREAKING’ GENE THERAPY: ‘LIKE A MIRACLE’
“The market is far smaller for rare diseases like SPG50, making it financially much less viable for corporations to put money into making a remedy.”
To commit himself to the trigger, Pirovolakis give up his job and began a nonprofit in California, which now has 5 staff and 20 consultants.
The corporate — referred to as Elpida Therapeutics, after the Greek phrase for “hope” — will run a Section 3 research for SPG50 on the NIH in November.

Terry Pirovolakis, second from left, is pictured with members of his group at his nonprofit, Elpida Therapeutics. Elpida Therapeutics has partnered with the Columbus Kids’s Basis (Fundación Columbus in Spain) and CureSPG50 to assist save youngsters with the illness. (Pirovolakis)
With out the backing of major drug companies, nevertheless, there isn’t funding out there to get the therapies to the kids who want them.
Eight doses of the drug for SPG50 have been produced in Spain and have been flown to the U.S.
“The remedy is right here, simply actually sitting in a fridge, able to go,” Lockard stated. “Medical doctors are prepared. There simply is not sufficient cash to make it occur.”
For more Health articles, visit www.foxnews.com/health
There are at present 4 households within the U.S. who’re attempting to boost the cash that is wanted, in response to Pirovolakis.
“Time is of the essence,” he stated. “We need to make sure that the trial strikes on and these youngsters get handled.”
The tip aim
Looking forward to the Section 3 medical trial on the NIH, Pirovolakis’ aim is to deal with eight youngsters with SPG50.
“If we are able to present that it really works in all eight youngsters — and we are able to show to the FDA that it’s making a distinction — then the drug will get authorized and each baby can get it,” he stated.

Michael Pirovolakis is pictured strolling with the help of a walker. Spastic paraplegia 50 (SPG50) is a neurological dysfunction that impacts a baby’s growth, progressively resulting in cognitive impairment, muscle weak point, speech impairment and paralysis. (Terry Pirovolakis)
Ideally, after the drug is authorized — which may take three to 5 years, Pirovolakis estimates — SPG50 will probably be added to hospitals’ newborn screening programs and each baby with the illness will have the ability to get the remedy.
Elpida Therapeutics has partnered with the Columbus Kids’s Basis (Fundación Columbus in Spain) and CureSPG50 to assist save youngsters with the illness.
CLICK HERE TO GET THE FOX NEWS APP
“Our partnership with Elpida is pushed by an unwavering dedication to leaving no baby behind,” Sheila Mikhail, co-founder of the CCF, stated in an announcement to Fox Information Digital.
“On the Columbus Kids’s Basis and Fundacion Columbus, as a world group, we imagine that each baby deserves an opportunity for a healthy future. Collectively, we’re making groundbreaking strides in treating ultra-rare genetic issues, making certain that no baby is left to face these challenges alone.”
“The most important problem in offering remedy for youngsters with uncommon illnesses typically comes right down to a scarcity of funding and imaginative and prescient.”
Pirovolakis stated he will get a number of calls every week from households around the globe, asking for assist saving their youngsters.
“Sadly, the largest problem in offering remedy for youngsters with uncommon illnesses typically comes right down to a scarcity of funding and imaginative and prescient,” he instructed Fox Information Digital.
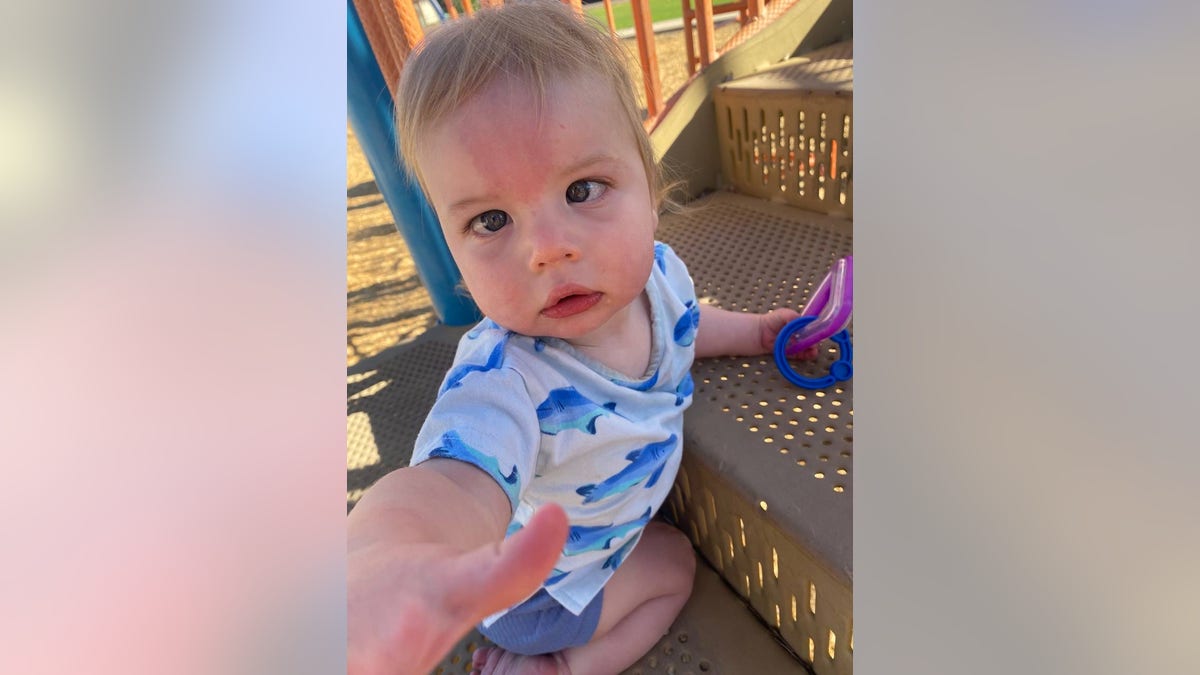
After Jack Lockard, pictured, obtained the gene remedy at 6 months previous, the household quickly observed enhancements in his cognitive and bodily milestones. (Rebekah Lockard)
“The technology to remedy our kids is already right here. I hope that somebody with immense wealth — and extra importantly, the imaginative and prescient and affect — will step in,” he stated.
“Their help couldn’t solely impression a handful of illnesses and kids, however lengthen hope to hundreds of uncommon illnesses and tens of millions of kids, each this technology and the following.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
At present, 40 million People reside with a uncommon illness, and one in 10 will suffer from a probably treatable uncommon situation.
Pirovolakis added, “Somebody or love will seemingly be affected by a uncommon illness.”

